As I explained to Martin yesterday, the conversion of heptanal to ethyl heptonoate requires multiple reactions. The first reaction involves the conversion of the alkyl aldehyde heptanal to the higher alcohol heptanol (CH
3(CH
2)
5CH
2OH or C
7H
16O). The conversion of heptanal to heptanol results in the production of the carboxylic acids heptanoic acid and 2-hydroxyheptanoic acid. Anyone who has read "Have You Seen Ester?" knows that a carboxylic acid is an acid whose formula ends in COOH, which is also known as a carboxyl group.
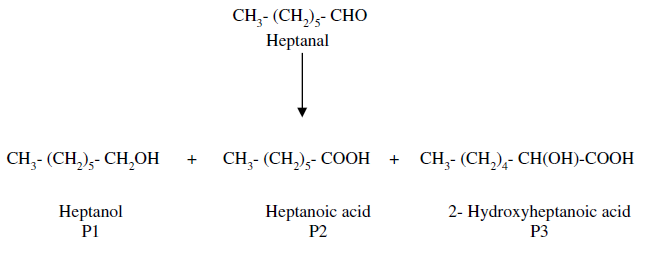
As one can clearly see, the formulas for heptanoic acid and 2-hydroxyheptanoic acid both end in COOH.
Ethyl heptanoate is the condensation reaction between ethanol and heptanoic acid.
C
2H
6O + CH
3(CH
2)
5COOH → C
9H
18O
2 + H
2O
The above reaction reads one molecule of ethanol plus one molecule of heptanoic acid yields one molecule of ethyl heptanoate plus one molecule of water.
I have the links to the publications that I provided to Martin if anyone is interested.